Scientists Capture the Heart’s Initial Formation
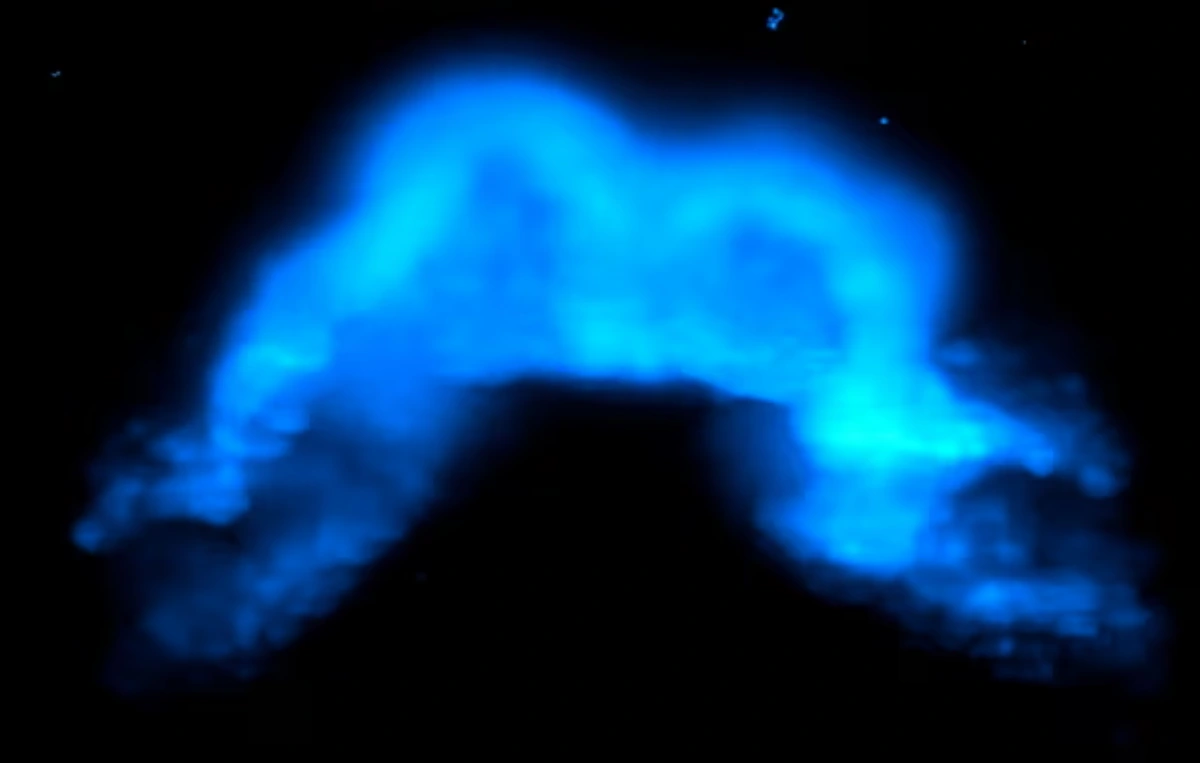
For the first time, scientists have captured images of the exact moment when cells in a mammalian embryo begin transforming into heart tissue. Using light-sheet microscopy on live mouse embryos, the team led by researcher Kenzo Ivanovitch from University College London tracked heart formation in real time—from the early stages of gastrulation to the development of the primitive heart tube.
The study, published in The EMBO Journal, reveals that the heart’s first progenitor cells not only emerge independently but also follow distinct migration paths that determine whether they will form specific parts of the heart, such as the left ventricle or the atria.
Directed Cell Migration from the Onset of Gastrulation
During gastrulation—a crucial embryonic process in which the body’s fundamental tissues begin to form—mesoderm cells migrate across long distances within the embryo. Until recently, this movement was thought to be disorganized. However, the new study shows that there are distinct patterns guiding these cells toward their cardiac fates.
“The migration paths were already linked to the cells’ final fate. Those that would give rise to the left ventricle, for example, followed more linear and coordinated routes,” Ivanovitch explained. “This suggests that the heart begins to take shape long before its visible structure emerges.”
Advertisement - Continue Reading Below
Two Distinct Groups of Cardiac Progenitors
By tracking more than a thousand cells in embryos cultured for up to 40 hours, the researchers identified two main groups of progenitors: those that form the left ventricle and atrioventricular canal (LV/AVC), and those that will give rise to the atria. The first group emerges early and differentiates within about 15 hours; the second appears later and is recruited during the folding of embryonic tissue that will eventually shape the heart.
“These two sets of cells not only originate at different times but also follow distinct routes within the embryo,” explained Shayma Abukar, the study’s first author. “It’s as if the heart is assembled in phases, with pieces arriving from different places and times.”
Multipotent Cells and Early Organization
The study also revealed the presence of multipotent progenitors—cells still capable of developing into more than one cell type—in the mesoderm region. However, this plasticity is short-lived. Most cells become specialized soon after their first or second cell division, indicating that cardiac fate is determined very early in development.
In complementary experiments, cells destined to form the pericardium and endocardium displayed different migration speeds and patterns, reinforcing the idea of functional organization already in the early embryonic phase. “We were surprised to see that cells with different cardiac fates not only behave differently but also either maintain contact with one another or quickly separate depending on their specialization,” Abukar noted.
New Line of Fluorescent Mice Enabled Cell Tracking
To capture the images, the researchers engineered a transgenic mouse line that expresses a fluorescent protein exclusively in cardiac cells (cTnnT-2a-eGFP). This modification allowed them to observe, in real time, where and when these cells began to differentiate within the embryo.
The use of light-sheet microscopy—a technique that minimizes tissue damage and allows for extended imaging sessions—was crucial. “We were able to capture the moment when a cell was still migrating and suddenly activated cardiac genes. It’s literally the birth of a heart cell,” Ivanovitch explained.
Relevance to Human Development and Regenerative Medicine
Although conducted in mice, the study offers crucial insights into heart development in mammals, including humans. Understanding the earliest steps of heart formation may help identify the causes of congenital malformations and improve techniques for generating cardiac cells in the lab.
“Our work paves the way for more precise studies on how to produce specific cardiac cells—such as those of the ventricle or atrium—from stem cells,” says Ivanovitch. “This has important implications for regenerative therapies and tissue bioengineering.”