New approach to spinal cord injury
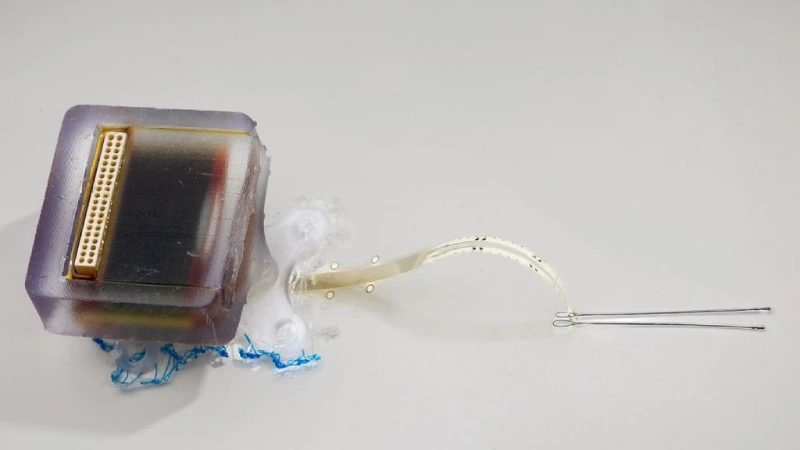
A study published in Nature Communications introduces a promising innovation for treating spinal cord injuries. Scientists from the University of Auckland (New Zealand) and Chalmers University of Technology (Sweden) have developed an ultrathin implant that, by applying daily electrical fields, significantly improved motor and sensory recovery in rats with thoracic spinal cord injuries. The treatment caused no inflammatory side effects and remained stable over a 12-week period.
Spinal cord injuries disrupt communication between the brain and the body, resulting in the loss of movement, sensation, and even autonomic functions such as bladder control. There is no definitive cure, and even small improvements can make a big difference in patients’ quality of life. Electrical stimulation is already used in medicine, but its effectiveness is still limited by technical issues such as corrosion of metal electrodes and difficulty in reaching deep areas of the spinal cord.
In this new study, researchers used a thin-film implant equipped with iridium oxide electrodes (SIROF), placed directly beneath the dura mater—the protective layer surrounding the spinal cord. This subdural position allows the electric field to penetrate more effectively into the nervous tissue, even with low-intensity currents. This reduces the risk of damage and improves the precision of the stimulation.
The tests were conducted on rats with a controlled injury between the L1 and L2 segments of the spinal cord. The treated group received daily one-hour sessions of electrical stimulation using 250-millisecond pulses at 2 hertz. Another group with the same injury received no treatment and served as a control. A third group, with no injury, provided a comparison with healthy animals.
The results were clear. From the fourth week onward, the treated rats outperformed the untreated ones in motor function tests, using the BBB scale, which assesses movement in the hind limbs. By the 12th week, all treated rats scored above 14, indicating coordinated movement between the front and hind limbs. In the control group, only 20% reached that level.
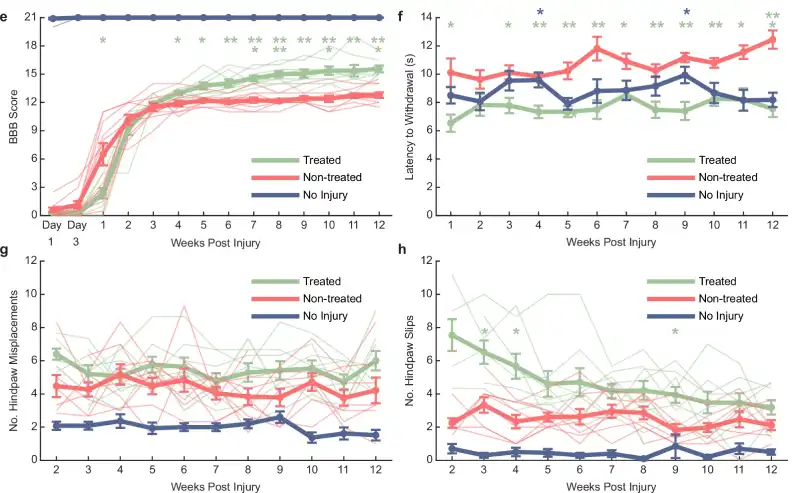
Touch sensitivity also improved in the treated animals. A mechanical filament test showed that they responded more quickly to stimuli on their paws, indicating early sensory recovery — already noticeable in the first week of treatment.
Interestingly, histological analysis of the injured tissue did not show an increase in axon density or signs of regeneration, suggesting that the functional improvement may have occurred through alternative mechanisms, such as protection of nerve cells or reorganization of existing neural connections.
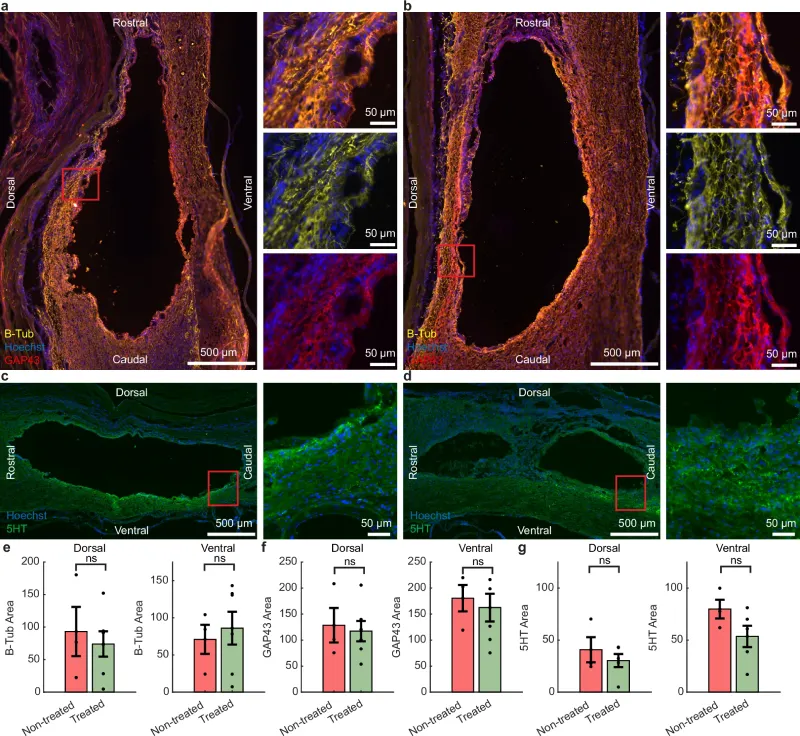
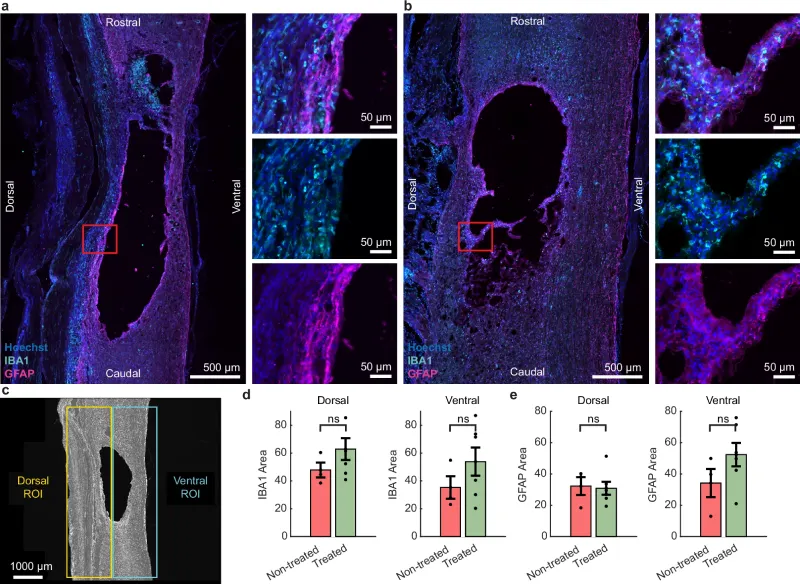
Another positive finding was the body’s tolerance to the implant. By the end of the experiment, no signs of inflammation or rejection were observed in the tissues in contact with the electrodes. Computer simulations confirmed that the subdural configuration allows for a more efficient distribution of the electric field in the spinal cord, using less energy and with a lower risk of adverse reactions.
In bench tests, the SIROF electrodes demonstrated stability even after 90 hours of continuous stimulation, with no signs of degradation. This reinforces their potential for long-term use in clinical applications.
Despite the promising results, the researchers caution that further studies are still needed, particularly to determine the optimal timing and intensity of the stimulation. They also plan to investigate whether the treatment can produce lasting effects even after the stimulation is stopped.
The technique represents a significant advancement and could, in the future, contribute to the development of regenerative therapies for humans with spinal cord injuries. While subdural implants are more invasive, they offer important advantages in terms of efficacy and safety.