How is good cholesterol produced?
High-density lipoproteins (HDL), also known as “good cholesterol,” remove excess cholesterol from body tissues and transport it to the liver. This process is known to prevent atherosclerosis, the buildup of plaque on artery walls. Atherosclerosis is associated with potentially fatal symptoms, including heart attacks, strokes, aneurysms, and blood clots. Despite the importance of HDL, scientists still have a limited understanding of how it is produced.
“Historically, it was believed that HDL removed excess cholesterol from cells by passive diffusion,” explains lead researcher Professor Kazumitsu Ueda of the Institute for Integrated Cell-Material Sciences (iCeMS) at Kyoto University. “However, in 1999, a genetic analysis of Tangier disease, a condition characterized by low levels of HDL in the blood, revealed that the ATP-dependent transporter protein ABCA1 was essential for HDL production. This only deepened the mystery—how was HDL produced, and what exactly did it do?”
Now, a team of researchers at iCeMS has used a new imaging method to reveal the molecular mechanism by which HDL is formed. They have shown how ABCA1 generates HDL molecules.
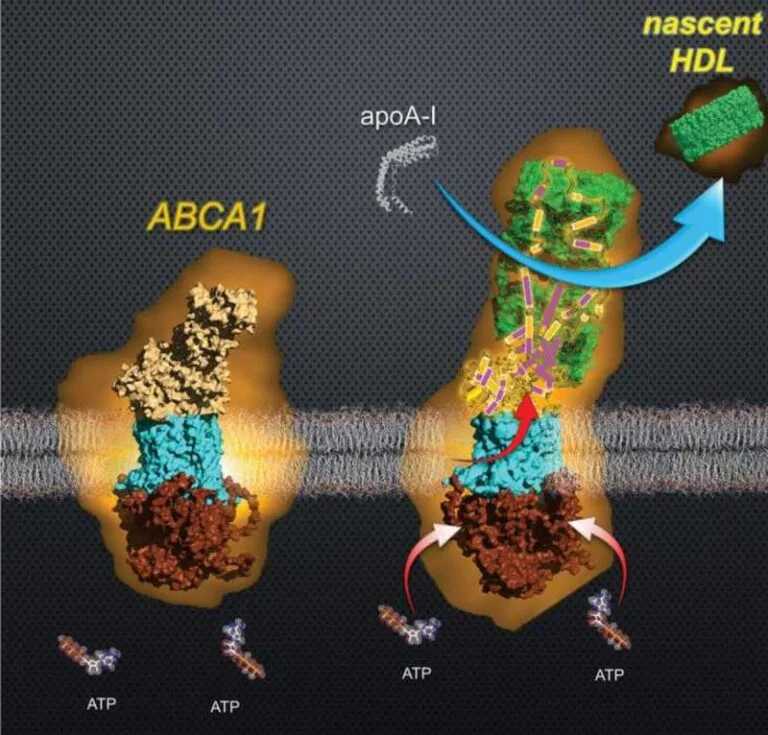
The team initially hypothesized that ABCA1 would temporarily store about 500 molecules of cholesterol and phospholipids in its extracellular domain (ECD), the part of ABCA1 that extends outside the cell. Although the ECD in ABCA1 is particularly large, an initial study using cryo-electron microscopy reported that the ECD of ABCA1 forms a “tunnel” that could only accommodate less than ten lipid molecules at a time.
To investigate this microscopic mystery, Atsushi Kodan, from Ueda’s team, worked together with Noriyuki Kodera’s team at the Institute for Life Science at the Nanoscale (Nano-LSI) at Kanazawa University, which developed the high-speed atomic force microscopy used in this study. This technique allows researchers to observe molecular processes with sub-second temporal and nanosecond spatial resolutions. “Few research groups in the world could perform this experiment,” says Ueda.
They observed that HDL generation is a much more complex process, in which ABCA1 transfers lipids to ECD through ATP hydrolysis, which releases the chemical energy stored in ATP bonds. ECD temporarily forms a new structure to store a large amount of lipids, which are then loaded en masse into apolipoprotein A-I (apoA-I). During this process, ECD undergoes a sudden and dynamic restructuring, losing about 30% of its volume. The loading of lipids into apoA-I produces the initial (nascent) HDL.
“The physiological roles of HDL and cholesterol are often not fully understood,” says Ueda. “By clarifying the function and regulatory mechanisms of ABCA1, we hope to promote a more accurate understanding.”
- See also: How staph bacteria latch onto human skin
The team hopes that a better understanding of how HDLs are produced and what functions they perform will help studies focus on the role of “good” and “bad” cholesterol in the body and inform the treatment of cholesterol-related diseases.
Kodan says that the high-speed atomic force microscopy used by the team allowed them to take side images of membrane proteins, something that is rare and difficult to do. “This new methodology for efficient lateral imaging of human ABCA1 has the potential to be applied to a wide range of membrane protein systems, including the transport of lipids, drugs, and metabolic products,” he says.
Additional information: https://doi.org/10.1021/acs.nanolett.5c03116