Nanotechnology reverses Alzheimer’s symptoms in mouse tests
A promising breakthrough in Alzheimer’s treatment has been achieved by an international team of researchers co-led by the Institute for Bioengineering of Catalonia (IBEC) and the West China Hospital of Sichuan University (WCHSU). The group managed to reverse the disease in mice after just three injections with nanoparticles, according to a study published in the journal Signal Transduction and Targeted Therapy.
Unlike traditional approaches that focus on neurons and other brain cells, the new treatment works by restoring the normal function of the brain’s vasculature—an essential element for brain health. The innovative strategy uses bioactive nanoparticles, known as “supramolecular drugs,” which, instead of merely delivering medication, act directly on the blood-brain barrier (BBB), the structure responsible for protecting the brain and regulating its internal environment.
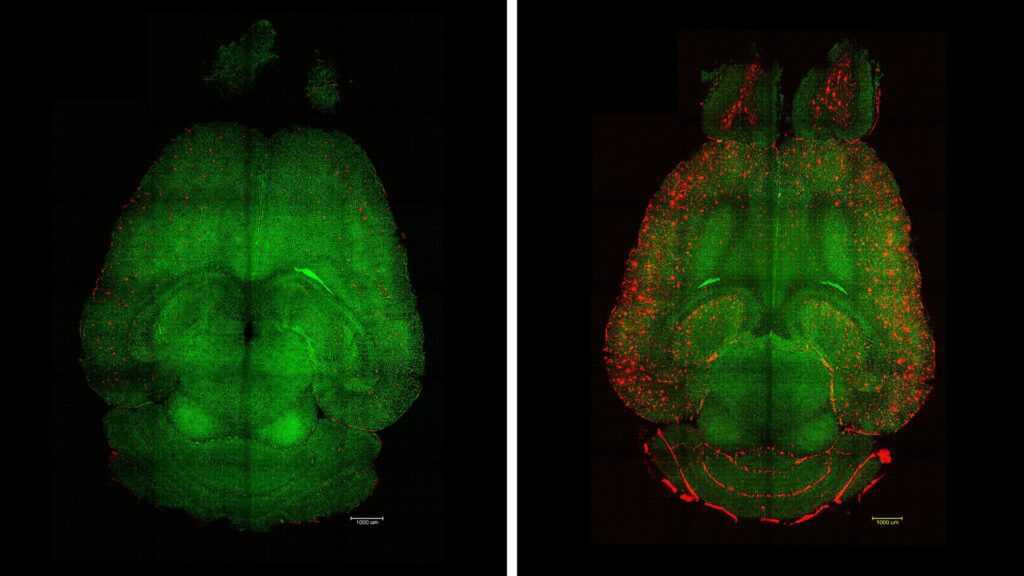
The results were remarkable: just one hour after administration, researchers observed a 50% to 60% reduction in the amount of beta-amyloid (Aβ) protein—the main culprit behind neuronal damage in Alzheimer’s. “Only one hour after the injection, we noticed a drastic decrease in Aβ levels in the brain,” explained Junyang Chen, co-author of the study and researcher at Sichuan University.
The blood-brain barrier acts as a filter, preventing pathogens and toxins from entering the brain. However, in neurodegenerative diseases, this system deteriorates, making it harder to clear toxic proteins like Aβ. The nanoparticles developed by the scientists “reprogram” this barrier, allowing the brain to naturally resume the elimination of harmful substances.
According to Giuseppe Battaglia, professor at IBEC and lead researcher, the treatment showed a lasting effect by restoring the health of brain blood vessels:
“The long-term effect comes from the recovery of the brain vasculature. Our nanoparticles seem to activate a feedback mechanism that restores the brain’s cleaning system to normal levels.”

The experiments were conducted on genetically modified mice designed to develop symptoms similar to those of human Alzheimer’s disease. One of the tests involved a 12-month-old mouse—equivalent to a 60-year-old human—treated with the nanoparticles. Six months later, the now-aged mouse displayed behavior similar to that of a young and healthy animal.
At the molecular level, the treatment acts on the LRP1 protein—a sort of “gatekeeper” that transports Aβ from the brain into the bloodstream. When this system fails, the toxic protein accumulates, worsening the disease. The new supramolecular drugs mimic LRP1 ligands, allowing Aβ to be efficiently removed and restoring the brain’s natural balance.
Moreover, the design of the nanoparticles—achieved through high-precision molecular engineering—enables exact control over their size and the number of ligands, improving their interaction with the cells of the blood-brain barrier. This paves the way for a new generation of smart nanomedicine therapies capable of modulating cellular functions in a targeted manner.
“Our study demonstrated remarkable efficacy in rapidly eliminating Aβ, restoring healthy blood-brain barrier function, and impressively reversing Alzheimer’s pathology,” said Lorena Ruiz Pérez, researcher in the Molecular Bionics group at IBEC and professor at the University of Barcelona.
Advertisement - Continue Reading Below

The study brought together scientists from IBEC, Sichuan University, University College London, the University of Barcelona, the Chinese Academy of Medical Sciences, and the Catalan Institution for Research and Advanced Studies (ICREA).
With this discovery, the researchers open a new horizon for Alzheimer’s treatment, focusing on brain vascular health—a previously underestimated factor—and offering hope for the development of effective therapies against one of today’s most devastating neurodegenerative diseases.
🧬 Reference:
Chen, J. et al. Rapid amyloid-β clearance and cognitive recovery through multivalent modulation of blood–brain barrier transport. Signal Transduction and Targeted Therapy, 2025. DOI: 10.1038/s41392-025-02426-1