Brain circuit may be the key to treating panic disorder

For those who have experienced the gripping terror of a panic attack, the symptoms are all too familiar – overwhelming fear, sweaty palms, shortness of breath, and a racing heartbeat. These episodes, which can strike unexpectedly, are the hallmark of panic disorder, a condition that affects millions worldwide.
Despite its prevalence, the underlying brain mechanisms driving panic attacks have remained elusive, hindering the development of effective targeted treatments. But a groundbreaking study by researchers at the Salk Institute has shed new light on this enigmatic disorder, revealing a specific brain circuit that could hold the key to unlocking novel therapeutic approaches.
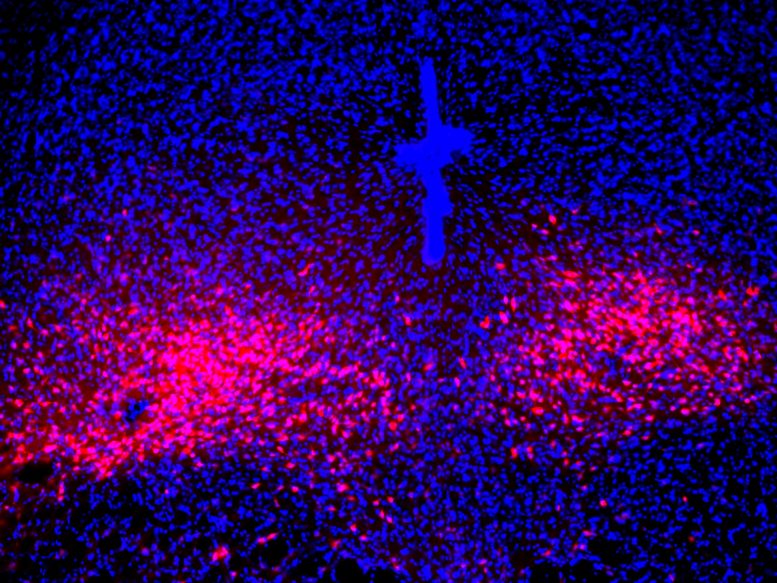
The study, led by associate professor Sung Han, has mapped a previously unknown neural pathway that appears to play a pivotal role in mediating panic-like symptoms in mice. This discovery challenges the long-held assumption that the amygdala, often dubbed the brain’s fear center, is solely responsible for panic attacks. Instead, Han’s team has identified a distinct circuit centered in the lateral parabrachial nucleus (PBL), a small brainstem region known as the brain’s alarm center.
The PBL’s strategic location and functions make it a compelling candidate for orchestrating panic attacks. This tiny area not only controls vital functions like breathing, heart rate, and body temperature but also produces a neuropeptide called PACAP (pituitary adenylate cyclase-activating polypeptide), a master regulator of stress responses.

“We’ve been exploring different areas of the brain to understand where panic attacks start,” says senior author Sung Han, associate professor at Salk. “Previously, we thought the amygdala, known as the brain’s fear center, was mainly responsible—but even people who have damage to their amygdala can still experience panic attacks, so we knew we needed to look elsewhere. Now, we’ve found a specific brain circuit outside of the amygdala that is linked to panic attacks and could inspire new panic disorder treatments that differ from currently available panic disorder medications that typically target the brain’s serotonin system.”
To unravel the intricate workings of this circuit, the researchers turned to a mouse model of panic attacks, allowing them to observe the real-time activity of PACAP-expressing neurons. Their findings painted a vivid picture: During a panic attack, these neurons became activated, releasing PACAP messengers that traveled to another brain region called the dorsal raphe, where neurons expressing PACAP receptors reside. Upon receiving these signals, the receptor neurons triggered the manifestation of panic-associated behavioral and physical symptoms in the mice.
This discovery not only illuminates the intricate neural circuitry underlying panic attacks but also highlights a potential therapeutic target. By inhibiting PACAP signaling, the researchers could disrupt the flow of these neuropeptide messengers, effectively reducing panic symptoms in the mice – a promising avenue for the development of panic disorder-specific treatments.
“Emotional and stress-related behaviors have been associated with PACAP-expressing neurons in the past,” says co-first author Sukjae Kang, senior research associate in Han’s lab. “By mimicking panic attacks in the mice, we were able to watch those neurons’ activity and discover a unique connection between the PACAP brain circuit and panic disorder.”
Sung Han, the study’s senior author, emphasizes the importance of this discovery, noting the distinct differences between panic disorder and other anxiety-related conditions. “Despite panic disorder’s categorization as an anxiety disorder, there are many ways that anxiety and panic are different,” he explains. “Panic induces many physical symptoms, like shortness of breath, pounding heartrate, sweating, and nausea, but anxiety does not induce those symptoms. Or how panic attacks are uncontrollable and often spontaneous, while other anxiety disorders, like post-traumatic stress disorder (PTSD), are more memory-based and have predictable triggers.”
These distinctions underscore the need for tailored therapeutic approaches, and the PACAP brain circuit presents a promising target. “We found that the activity of PACAP-producing neurons in the brain’s parabrachial nucleus is inhibited during anxiety conditions and traumatic memory events,” Han notes. “Because anxiety seems to be operating conversely to the panic brain circuit, it would be interesting to look at the interaction between anxiety and panic, since we need to explain now how people with anxiety disorder have a higher tendency to experience panic attack.”
The team’s findings have far-reaching implications, not only for panic disorder but also for our understanding of the intricate interplay between anxiety and panic. By mapping the PACAP circuit, they have opened a new window into the brain’s intricate workings, revealing a previously unknown pathway that could hold the key to unlocking more effective treatments for panic disorder.
But the journey is far from over. The researchers are eager to further expand their map, exploring where the PACAP receptor-producing neurons in the dorsal raphe send their signals and how other anxiety-related brain areas interact with the PACAP panic system. This comprehensive understanding could pave the way for targeted therapies that address the unique neurological underpinnings of panic disorder, offering hope for those grappling with this debilitating condition.
As the team continues to unravel the mysteries of the panic brain circuit, their findings represent a significant stride toward a future where panic attacks are no longer an enigma but a treatable condition with targeted, effective interventions. By illuminating the intricate neural pathways that underlie these harrowing episodes, they are paving the way for a new era of precision treatments, offering relief and newfound hope to those living with panic disorder.
The findings were published in the journal Nature Neuroscience.






