Human eggs created from skin cells
Infertility affects millions of people worldwide and is often associated with the absence of functional gametes. In women, the progressive decline in the quality and quantity of oocytes after the age of 35 is one of the main factors.
For patients without viable gametes, conventional in vitro fertilization (IVF) treatments are ineffective, leaving donor use as the only alternative. In this context, in vitro gametogenesis (IVG) emerges as a promising strategy, allowing eggs or sperm to be obtained from the patient’s own somatic cells.
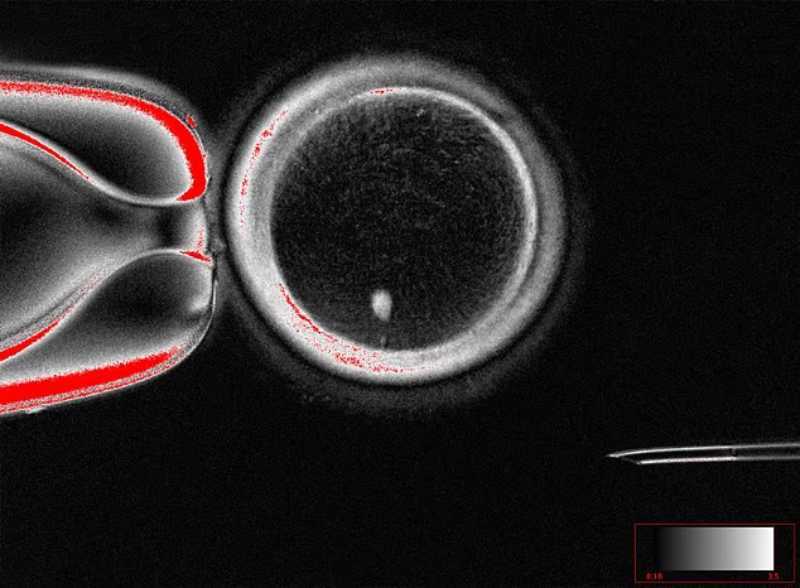
A recent study published in Nature Communications proposed a remarkable advance in this field by exploring an experimental technique called mitomeiosis. The work, led by Shoukhrat Mitalipov and colleagues, investigated how somatic cell nuclear transfer (SCNT) could be adapted to induce cell divisions capable of reducing chromosomal ploidy, an essential requirement for the formation of functional gametes.
During natural reproduction, human gametes undergo a process of meiosis that reduces the number of chromosomes by half, ensuring that the fusion between egg and sperm restores diploidy (46 chromosomes). However, when somatic cell nuclei are transferred to enucleated oocytes, as occurs in SCNT, the initial result is a diploid embryo. This makes natural fertilization unfeasible, as it would lead to a condition of triploidy.
Mitomeiosis was developed precisely to circumvent this problem. The technique consists of forcing a somatic nucleus in the G0/G1 state (2n2c), i.e., without prior DNA duplication, to prematurely enter metaphase within the cytoplasm of an enucleated human oocyte. Thus, the somatic genome is induced to undergo a “reductive” division similar to meiosis, albeit without all the natural mechanisms of recombination.
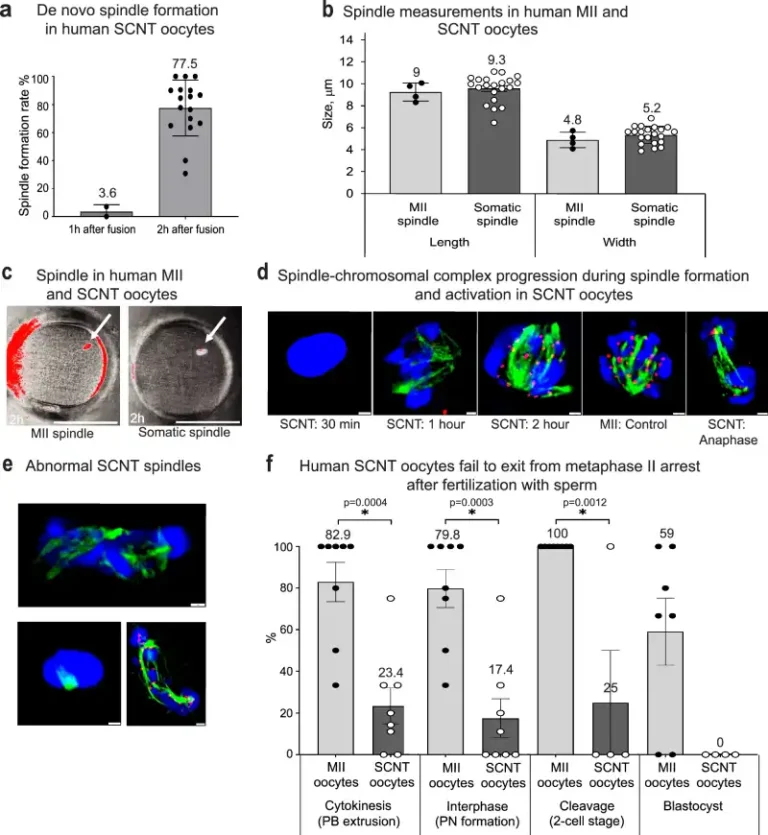
The researchers observed that oocytes reconstructed with somatic nuclei were able to form bipolar spindles but did not respond adequately to sperm-induced activation.
To overcome this limitation, it was necessary to resort to artificial activation through a combination of electroporation with roscovitine, a selective inhibitor of cyclin-dependent kinases. This protocol allowed the extrusion of polar bodies and the formation of pronuclei, signaling that chromosome segregation had occurred.
Detailed analysis of the chromosomal content revealed that, on average, 23 somatic chromosomes were retained in the zygote, a number equivalent to that expected for a human gamete. However, unlike natural meiosis, segregation was random and there was no recombination between homologous chromosomes. Even so, embryos derived from these zygotes were able to initiate mitotic divisions and, in some cases, reach the blastocyst stage.
Although the study demonstrates the feasibility of inducing artificial reductive division in human cells, several limitations still need to be overcome before the technique can have clinical applications. Among them are:
- Absence of meiotic recombination: essential for genetic diversity and correct chromosome segregation.
- Frequent aneuploidies: resulting from the random distribution of chromosomes.
- Low embryo development rate: only 8.8% of fertilized SCNT embryos reached the blastocyst stage.
- Possible epigenetic reprogramming failures: which may compromise long-term viability.
Nevertheless, the concept of mitomeiosis paves the way for advances in the production of artificial human gametes. If refined, this approach could offer new treatment alternatives for infertility, especially in cases where no gametes are available. In addition, it provides a valuable experimental model for investigating the fundamental mechanisms of meiosis and cellular reprogramming.
- See also: How staph bacteria latch onto human skin
🔬 Reference: Gutierrez, N. M. et al. Induction of experimental cell division to generate cells with reduced chromosome ploidy. Nature Communications (2025). DOI: 10.1038/s41467-025-63454-7