Scientists create human mini-brain with safety systems
For the first time, scientists have successfully created human mini-brain with built-in safety systems, marking a significant milestone in neurological research. These tiny organoids, about the size of a sesame seed, include functional blood-brain barriers (BBB). In a full-sized brain, the BBB plays a crucial role by protecting the delicate organ from potentially harmful substances circulating throughout the body.
“The lack of an authentic human BBB model has been a major obstacle to studying neurological diseases,” stated Ziyuan Guo, a neurobiologist and stem cell scientist at Cincinnati Children’s Hospital Medical Center. This innovation is particularly important as current animal models do not accurately reflect human brain development or BBB functionality, hindering the progress of neurological disease research.
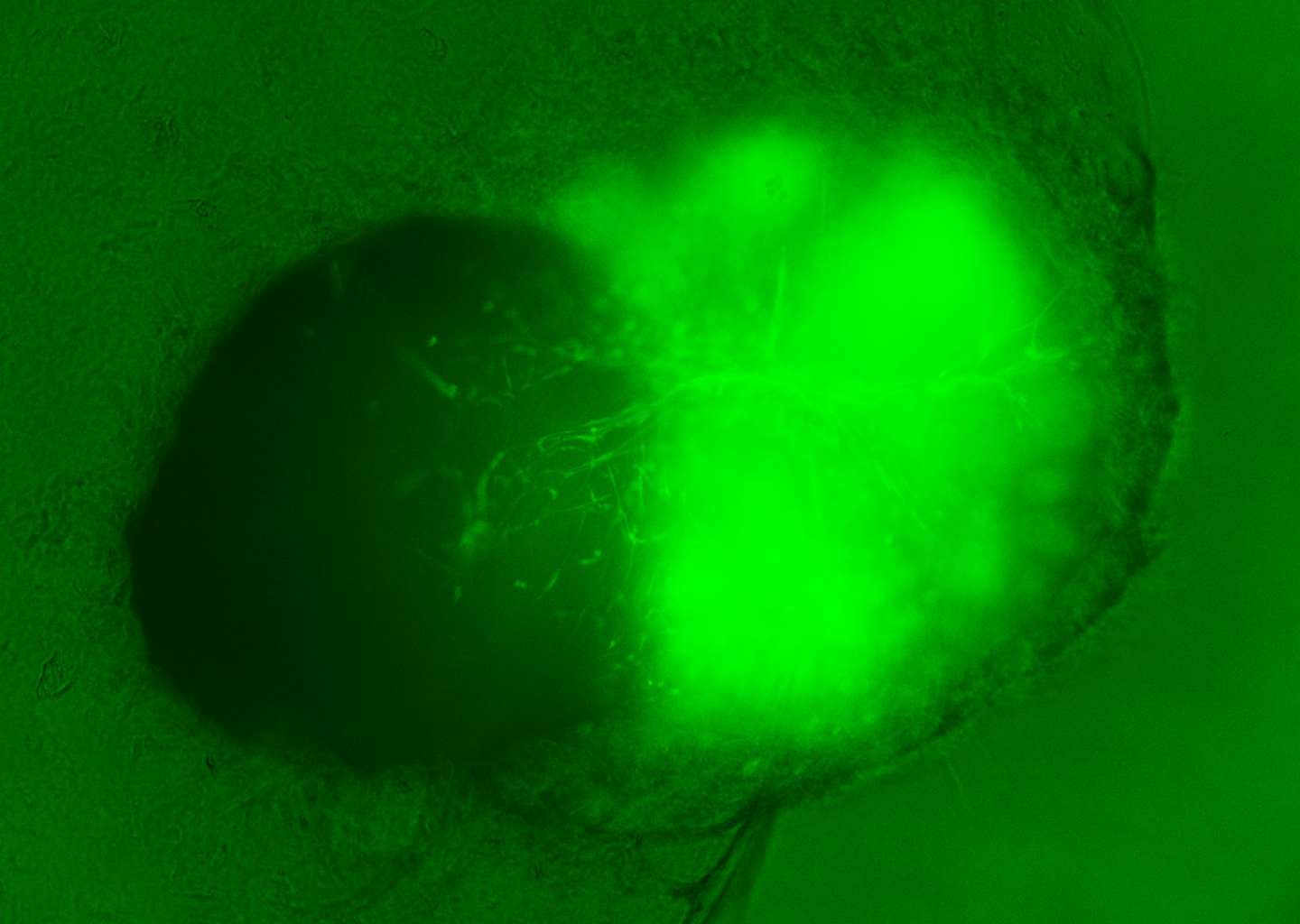
In the human body, the BBB lines the blood vessels running through the brain, selectively allowing certain substances, such as hormones and glucose, to pass through while blocking threats like toxins and bacteria. This barrier also impedes the passage of many drugs, posing a significant challenge for drug developers working on treatments for brain diseases.
The newly developed lab-grown models combine brain organoids—three-dimensional clusters of brain cells grown from stem cells—with blood vessel organoids, which are also stem cell-derived but resemble the body’s vasculature. Together, these two types of organoids form what researchers call “ensembloids,” which mimic the interaction and development of brain and blood vessel cells.

Approximately a month after being combined, these organoids coalesced into spherical structures, each about the size of a sesame seed. “The culture that supports the growth and fusion of the two organoids must be carefully controlled, and a physical gel matrix acts as a scaffold to help support the assemblies,” Ziyuan explained to Live Science.
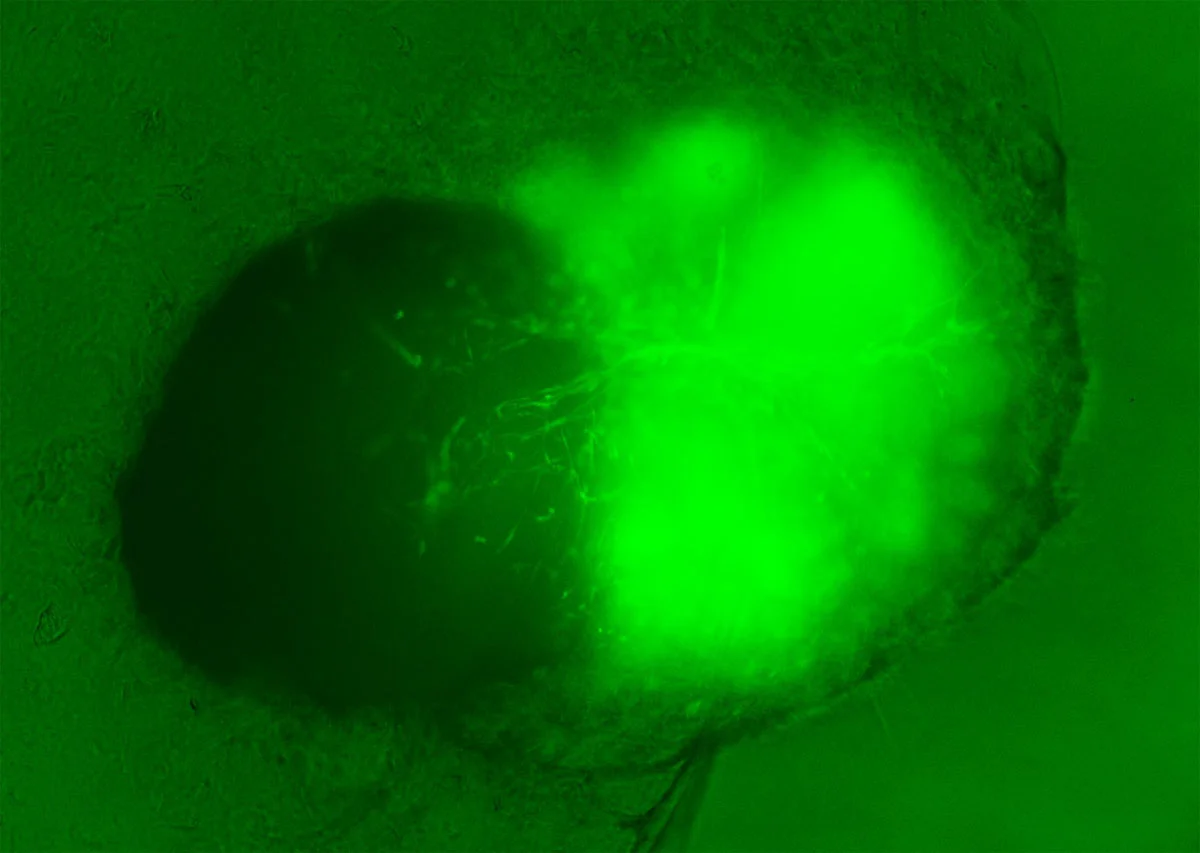
As a proof-of-concept, the researchers cultured assembloids with cells from patients with cerebral cavernous malformation, a condition characterized by clusters of abnormally formed blood vessels in the nervous system. This condition, often caused by genetic mutations, can lead to serious complications such as strokes and seizures.
The resulting assembloids effectively captured cellular features observed in individuals with these malformations, providing new insights into the molecular and cellular pathology of cerebral vascular disorders. This demonstrates the potential of these models to improve our understanding of neurological diseases and the development of effective treatments.
“In initial tests, assembloids with BBB can be grown for up to five months, or potentially longer, although this has not been tested,” Ziyuan specified. “Four to five months of growth roughly corresponds to the second trimester of brain development in utero.”

Looking ahead, the research team plans to grow similar assemblies using stem cells from individuals with different brain diseases, ensuring that the final models reflect the underlying biology of those conditions. This approach holds promise not only for studying brain diseases but also for investigating how toxins damage the brain and BBB. Additionally, these models could reveal new strategies for drug delivery, overcoming the challenge posed by the BBB’s selective permeability.
“BHE-enabled ensembloids represent a revolutionary technology with broad implications for neuroscience, drug discovery, and personalized medicine,” Ziyuan concluded. This breakthrough opens up new avenues for understanding and treating neurological diseases, offering a more accurate and efficient platform for drug testing and the study of brain development and disorders.
The creation of mini human brains with functional blood-brain barriers represents a transformative advancement in neuroscience. These innovative models bridge a significant gap in neurological research, providing a more accurate representation of human brain development and BBB functionality than existing animal models.
As researchers continue to refine these models and explore their potential applications, the future of neurological disease research and treatment looks promising. This pioneering work not only enhances our understanding of the brain’s intricate biology but also paves the way for new therapeutic approaches to combat brain diseases.
The study was published in the journal Cell Stem Cell.