In search of the world’s thinnest metal wire

Researchers at the École Polytechnique Fédérale de Lausanne (EPFL) in Switzerland have made a revolutionary discovery in the field of nanotechnology. They simulated thousands of one-dimensional (1-D) materials and found what may be the thinnest metal wire ever discovered. This wire is made up of an alternating chain of two carbon atoms and one copper atom. This discovery promises to open up new frontiers in the miniaturization and efficiency of electronic components.
One-Dimensional Materials:
One-dimensional materials, such as carbon nanotubes, are formed by stringing individual atoms together in a single line. They are highly prized because of their unique electrical, magnetic and optical properties, which are often unusual and extremely useful for advanced technological applications. The discovery of this new metal wire could be a significant milestone in this field.
The Discovery Process
Chiara Cignarella, the lead author of the study, together with her colleagues Davide Campi and Nicola Marzari, began by questioning whether computer simulations using already known crystal structures could identify the ideal material without the need to physically build them in the laboratory. They focused on three-dimensional crystals that could be easily exfoliated until only a one-dimensional chain of atoms remained. This method has previously been used to extract two-dimensional materials, such as graphene, but it would be the first time it had been applied to obtain one-dimensional materials.
Exfoliation and Specialized Algorithm
The conceptual approach that led to these discoveries was developed at EPFL’s Materials Theory and Simulation Laboratory, as part of the National Center of Competence in Research’s (NCCR) MARVEL effort.
The exfoliation process involves “peeling away” excess material from the original 3D structure until only the 1-D chain remains. To identify the most promising candidates, the researchers created a database of more than 780,000 known three-dimensional crystal structures, focusing specifically on crystals held together by van der Waals forces.
According to the press release announcing the discovery, van der Waals forces are “the sort of weak interactions that happen when atoms are close enough for their electrons to overlap.”
A specialized algorithm was developed to filter this vast amount of data. The algorithm identified crystals with the right atomic organization to form natural wires and calculated the energy needed to separate these nanowires from the base crystal, thus determining the feasibility of manufacturing them.
Promising results
Initially, the analysis reduced the potential crystals to 800 viable candidates. Subsequent refinements based on simulated stability and electronic and structural properties further reduced this list to 14, and finally to the four most promising candidates. Of these, two were metals and the other two were semi-metals.
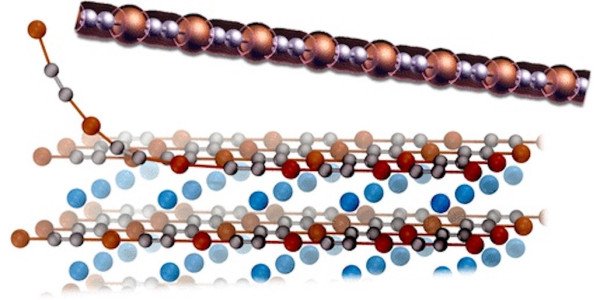
The most intriguing candidate identified by the algorithm was a linear chain made up of two carbon atoms and a copper atom, called the CuC2 metal wire. Simulations showed that CuC2 had the electronic conductive properties of a wire and remained stable at normal operating temperatures. Its resistance to Peierls distortions, which are breaks in the one-dimensional chain, was a crucial factor in its stability.
“It’s really interesting because you wouldn’t expect a real string of atoms along a single line to be stable in the metallic phase,” Cignarella said of his leading candidate.
Exfoliation from Different Sources
The researchers discovered that CuC2 wire could be exfoliated from three different crystals: NaCuC2, KCuC2 and RbCuC2. The extraction requires little energy, and the chain can be bent without losing its metallic properties, making it ideal for flexible electronics applications. The study’s press release explained the importance of exfoliating three different crystals to obtain CuC2: “It requires little energy to be extracted from them, and its chain can be [bent] while preserving its metallic properties, which would make it interesting for flexible electronics.”
Other promising discoveries
In addition to the CuC2 wire, the algorithm identified other promising one-dimensional materials. One of them, the semi-metal Sb2Te2, could enable advanced studies into excitonic insulators, an exotic state of matter theorized more than 50 years ago but never directly observed. If manufactured, Sb2Te2 could make this quantum phenomenon visible on macroscopic scales.
Next Steps and Future Implications
The next step for the research team is to collaborate with experimental scientists to synthesize these one-dimensional materials, including the copper carbon nanowire. Creating these materials in the real world will allow scientists to test how they carry electrical charges and how different temperatures affect their stability and performance. According to the study’s authors, “both aspects will be key to understanding how they would behave in real-world applications”.
The discovery of the thinnest metal wire ever created marks a significant advance in nanotechnology. This wire, composed of an alternating chain of carbon and copper atoms, could have wide applications in miniaturized and flexible electronic components. In addition, the innovative approach of using computer simulations to identify promising materials demonstrates the power of combining theory and simulation in advancing materials science.
The ability to exfoliate these wires from existing crystals with little energy opens up new possibilities for the manufacture of nanomaterials. This progress highlights the potential of advanced simulation techniques and specialized algorithms in the search for materials with novel and valuable properties, driving technological innovation to new heights.
The study was published in the journal ACS Nano.