Portable printer creates biodegradable implants to regenerate bones
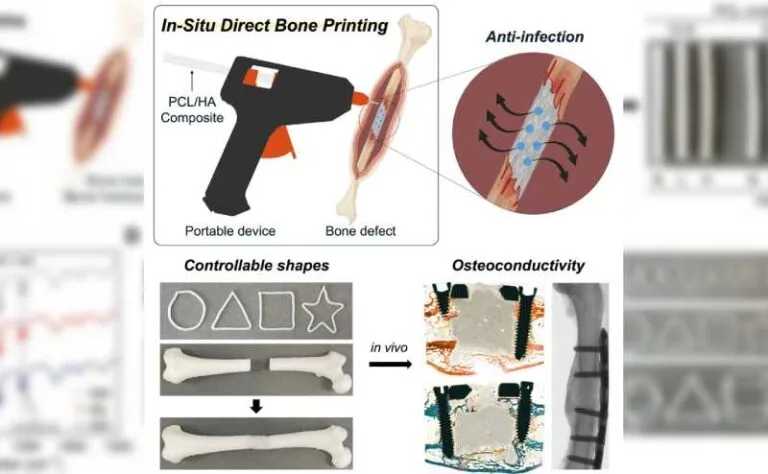
Regenerative medicine has just received a promising innovation: a portable device capable of printing biodegradable implants directly into large bone defects. The equipment, described in a study published in the journal Device, was developed through an international collaboration between researchers from South Korea, the United States, and other partner institutions.
Human bone has a remarkable capacity to regenerate in minor fractures, but when the injury reaches large dimensions—such as in severe accidents or surgeries for tumor removal—the natural healing process becomes insufficient. Current options include autologous grafts (taken from the patient’s own body), bone donations (allogeneic), or metal implants. All of these alternatives have limitations: risk of rejection, complications at the donor site, difficulty adapting to irregular geometries, and, in the case of metals, long-term integration problems.
In this context, 3D printing has been explored as a personalized solution for bone reconstruction. However, conventional methods require expensive equipment, pre-prepared components, and complex processing, which limits their clinical application.
In everyday use, the term “biodegradable” refers to materials that are quickly broken down by microorganisms, such as paper, organic waste, or certain plastics. In biomedicine, however, the concept is more specific: it refers to implants designed to degrade slowly within the body through natural chemical and enzymatic processes.
These implants function as a temporary scaffold: they support the injured area while the bone tissue regenerates and, over months or years, are gradually absorbed by the body into non-toxic byproducts, eliminating the need for surgery to remove them.
In the study, the chosen material was polycaprolactone (PCL), a biodegradable synthetic polymer, combined with hydroxyapatite (HA), a mineral that makes up a large part of the natural bone matrix.
The team led by Jung Seung Lee (Sungkyunkwan University, South Korea) and Giovanni Traverso (MIT and Harvard Medical School) created a device resembling a hot glue gun, capable of extruding the PCL and HA mixture directly at the site of the injury.
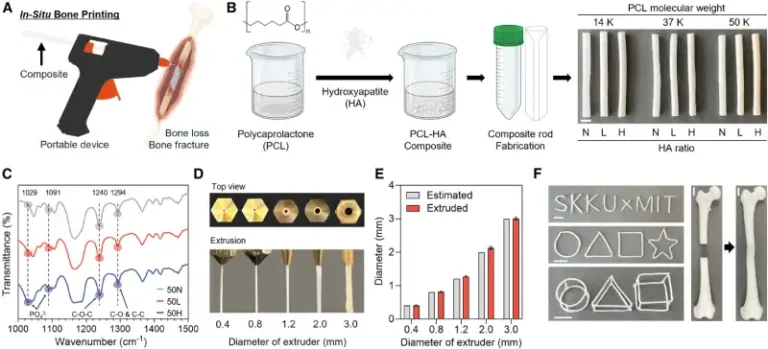
The low melting temperature of PCL (around 60 °C) makes it possible to print the material without causing damage to surrounding tissues, while the incorporation of HA increases mechanical strength and stimulates bone regeneration. The system also allows the addition of antibiotics to the mixture, providing protection against infections, one of the main risks in orthopedic surgeries.
The researchers demonstrated that it is possible to vary the amount of HA (0%, 12.5%, and 25%) and the molecular weight of PCL to control the implant’s strength, elasticity, and degradation time. Mechanical tests showed that HA-rich formulations withstand compression better and exhibit greater elasticity—properties essential for supporting body weight and movement.
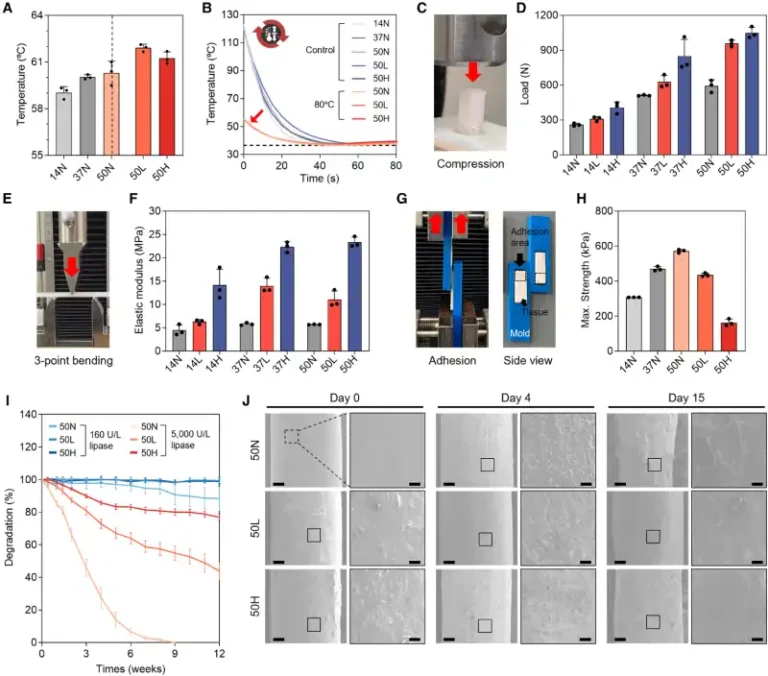
Degradation analysis revealed that implants containing HA degrade more slowly, maintaining stability for the time needed for natural bone to regenerate. Antibiotic assays showed gradual drug release and a bactericidal effect against Escherichia coli and Staphylococcus aureus, common microorganisms in postoperative infections.
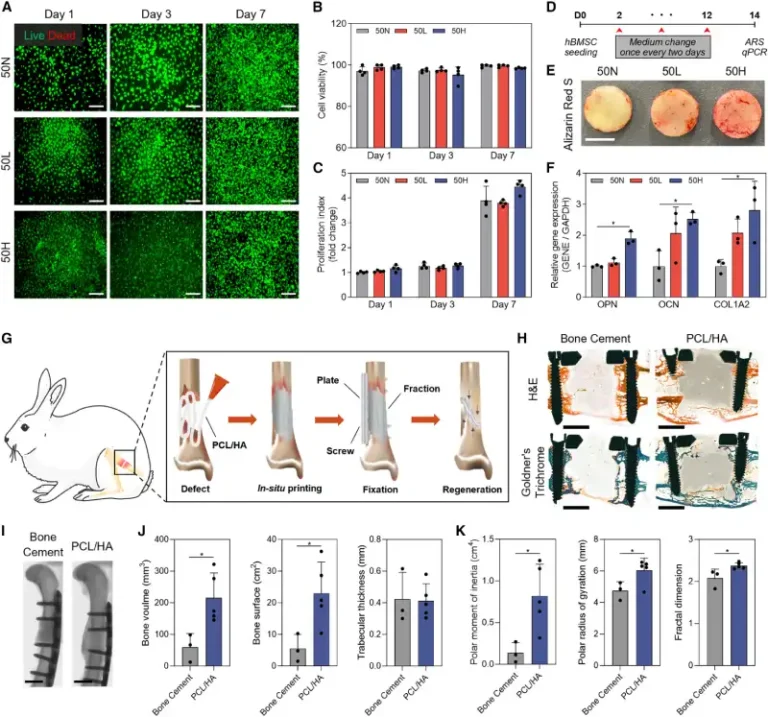
Tests with pre-osteoblastic cells and human mesenchymal stem cells confirmed that the scaffolds were non-toxic and promoted cell proliferation. Furthermore, the presence of HA stimulated differentiation into bone-forming cells, increasing calcium deposition and the expression of osteogenic markers.
The next step was to test the technology in an animal model. In rabbits with critical femoral defects, implants printed in situ (with a 25% HA formulation) showed greater bone tissue formation compared to commercial bone cements. After 12 weeks, histological and micro-computed tomography analyses revealed denser, more organized, and mechanically stable bones.
The authors emphasize that the device has the potential to transform orthopedic surgeries by providing personalized, rapid, and safer grafts. Its portability and ease of use allow doctors to customize implants directly at the injury site, without the need for prosthesis manufacturing facilities or lengthy digital planning processes.
Despite these advances, challenges remain before large-scale clinical application. These include optimizing implant adhesion to bone, improving extrusion parameters, and testing in larger animal models. The researchers also highlight the potential to incorporate other polymers, bioactive molecules, and systems that promote vascularization and accelerate healing.
If confirmed in clinical trials, in situ bone printing could represent a milestone in regenerative medicine, reducing complications, shortening recovery time, and improving the quality of life for patients with severe bone defects.
Additional information: Jeon, I.Y., Jeon, Y.M., Choi, J.H., et al. (2025). In situ printing of biodegradable implant for healing critical-sized bone defect. Device, 3, 100873. https://doi.org/10.1016/j.device.2025.100873