Microneedles that could revolutionize cancer immunotherapy
Cancer immunotherapy is based on harnessing the body’s own immune system to fight tumors; however, the effective and precise delivery of these treatments remains a major challenge. In this context, transdermal drug delivery via microneedles (MNs) emerges as a minimally invasive and promising solution.
A new review published in Glycoscience & Therapy highlights an innovative material for these microneedles: natural polysaccharides. Derived from plants, animals, and microorganisms, these sugars—such as hyaluronic acid and chitosan—exhibit high biocompatibility and biodegradability, in addition to having the ability to actively modulate the immune system.
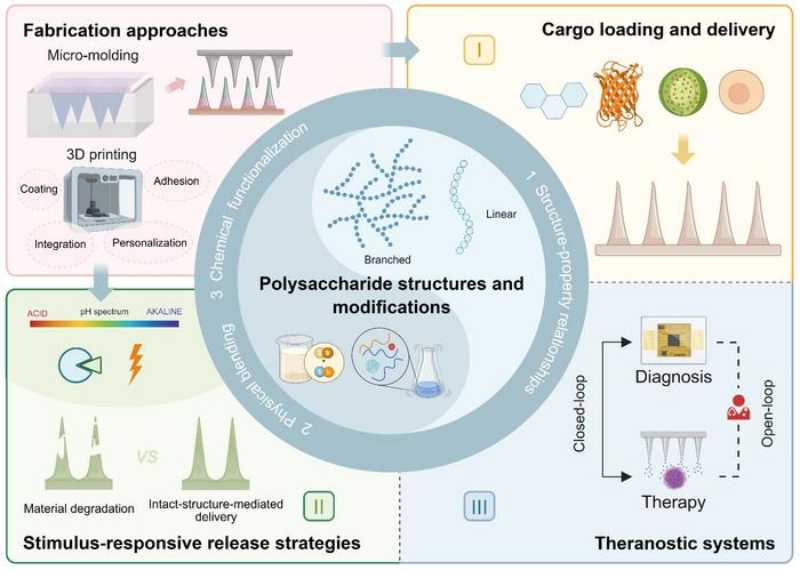
The distinguishing feature of this review lies in positioning polysaccharide-based microneedles (PMNs) as an integrated, active therapeutic platform rather than merely passive drug carriers. The authors analyze how PMNs function simultaneously as delivery vehicles and as immunomodulatory agents. The findings show that these microneedles can transport a wide range of anticancer agents—from small molecules and antibodies to nanoparticles—directly into the skin, leveraging the tissue’s rich network of immune cells. Beyond simply facilitating delivery, the polysaccharide matrix itself actively interacts with immune cells, enhancing the therapeutic response through a dual mechanism of action.
The review also details recent advances in materials science, summarizing the unique structure–activity relationships of polysaccharides, the possibility of fine-tuning their physicochemical properties, and how these features can be leveraged to enhance the mechanical strength, biocompatibility, controlled biodegradation, and tumor-specific responsiveness of microneedles.
Overall, the work highlights three interconnected innovations at the interface of materials and devices:
1. Dual-function design: The intrinsic bioactivity of natural polysaccharides (such as chitosan’s interactions with dendritic cells) endows PMNs with inherent immunomodulatory effects, generating synergistic responses when combined with incorporated anticancer agents.
2. Precision manufacturing frontier: Advanced fabrication techniques, particularly 3D printing, play a central role in overcoming the structural and functional limitations of conventional microneedles. These approaches enable the rational design of geometrically customized MN architectures with improved mechanical performance, tissue interaction, and drug-loading capacity—parameters that are essential for immunotherapy applications.
3. Microenvironment-responsive engineering: The review synthesizes strategies for developing PMNs that are sensitive to tumor-associated cues, such as pH gradients or enzymatic activity, enabling localized and temporally controlled drug release. This materials-guided adaptability represents a clear conceptual advance over static, non-responsive microneedle systems.
Taken together, this materials-driven perspective positions PMNs as a versatile and scalable platform for the next generation of cancer immunotherapy, providing a solid foundation for the rational design of intelligent drug–device combination systems in oncology.