This rare genetic mutation kills brain cells

The results raise the possibility that similar cell death pathways may also contribute to other neurological diseases, such as Alzheimer’s, Parkinson’s, or Huntington’s.
A team led by researchers at the German Helmholtz Centre in Munich discovered that mutations in a single gene cause neurons in mice to undergo progressive inflammation and cell death. In human brain cells grown in the laboratory, derived from the skin of patients with the same mutation, the neurons died in a surprisingly similar way. This specific form of programmed cell death is called ferroptosis, triggered by iron accumulation and oxidative damage to the cell membrane.
According to the researchers, the mechanism resembles cell death processes observed in dementia, based on the analysis of proteins expressed by neurons. Recent evidence, for example, indicates that ferroptosis is associated with Alzheimer’s disease.
In humans, this ultra-rare genetic disorder is known as Sedaghatian-type spondylometaphyseal dysplasia (SSMD), characterized by severe brain and skeletal abnormalities. The condition was first described in 1980, and since then, only a few dozen cases have been officially recorded, many involving children who died in the first few months of life.
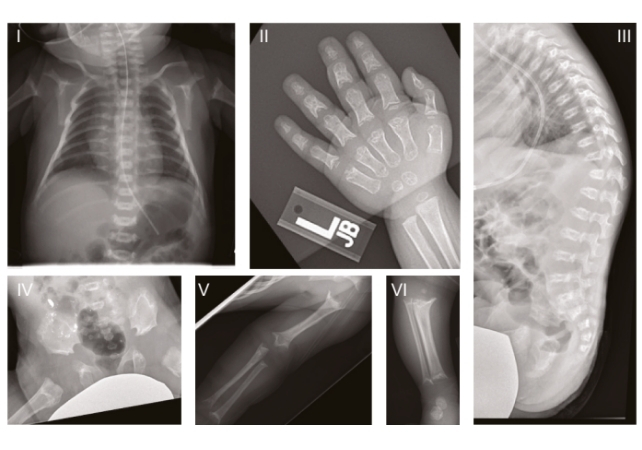
In recent years, genomic sequencing has linked SSMD to mutations in the gene that codes for the GPX4 enzyme , often considered a “guardian” of ferroptosis because it protects cell membranes against oxidative damage.
Although mutations in this gene do not necessarily lead to early-onset dementia, this new research, conducted on mouse cells and lab-grown “mini-brains,” reveals how GPX4 can protect neurons and how its dysfunction can result in cell death.
The study analyzed three children with SSMD in the United States, who presented with varying degrees of brain atrophy and mutations in the same functional region of the GPX4 gene. This data was then used in additional experiments with mice and with brain cells cultured from the skin of a patient with SSMD.
Marcus Conrad, a cell biologist and director of the Institute for Metabolism and Cell Death at Helmholtz Munich, compares the GPX4 enzyme to a surfboard.
“With its keel embedded in the cell membrane, it slides along the inner surface and rapidly neutralizes lipid peroxides as it moves forward,” he explains.
However, when this specific GPX4 mutation is present, the surfboard “keel” is absent. This means the enzyme cannot anchor to the membrane and therefore cannot protect the neuron. Neurons grown from stem cells of SSMD patients have proven particularly vulnerable to ferroptosis. Blocking this process, both in mice and in lab-grown cells, using a chemical compound, appeared to slow down neural death.
“Our data indicate that ferroptosis may be a driving force behind neuronal death — and not just a side effect,” says Svenja Lorenz, a cell biologist at Helmholtz Munich.
“Until now, dementia research has often focused on protein deposits in the brain, called beta-amyloid plaques. Now we are placing more emphasis on damage to cell membranes, which can initiate this degenerative process.”
Dementia is often considered a disease associated with old age, but in some tragic scenarios, cognitive decline linked to memory problems can begin much earlier. Childhood dementia is a rare condition that leads to memory loss and mental confusion, and genomic studies have linked it to more than 100 rare genetic disorders present from birth.
Investigating such tragic cases provides scientists with crucial information about how neurodegeneration can occur and what can be done to combat it.
“It took us almost 14 years to link a small, previously unrecognized structural element of a single enzyme to a serious human disease,” says Conrad.
“Projects like this clearly demonstrate why we need long-term funding for basic research and international, multidisciplinary teams if we truly want to understand complex diseases like dementia and other neurodegenerative conditions.”
The study was published in the journal Cell.